News Articles

Ben Martin Law Group Attorney Jessica Murray Earns Texas Rising Stars Selection
Elite Super Lawyers list highlights the best newer lawyers in the state HOUSTON – Jessica Murray, a lawyer with the Ben Martin Law Group in Dallas, has been named to the prestigious 2023 Texas Rising Stars legal guide by Super Lawyers. Attorneys who earn a place on this list must be 40 years…

Ben Martin Law Group Trial Lawyers Earn Top Verdict Honors for 2021 IVC Filter Litigation
Dallas trial firm stands out for successes in medical device defect litigation DALLAS – A $2.549 million jury verdict over a defective medical device secured by Ben Martin Law Group has been recognized as one of the state’s top 100 verdicts for 2021, according to an analysis by Texas Lawyer magazine and VerdictSearch. The May 2021 verdict…

Law Firm Honored as National Law Journal’s Elite Trial Lawyers Finalists
National award based on 2021 work in mass tort litigation DALLAS – Dallas-based Martin Baughman (now Ben Martin Law Group) was named 2022 finalist for the National Law Journal’s Elite Trial Lawyers honors for mass tort litigation based on the firm’s string of jury verdicts and settlements on behalf of individuals injured by defective medical…

Trial Lawyer Ben C. Martin Named to D Magazine’s ‘Best’ List
Repeat honors for nationally respected attorney Ben C. Martin for mass torts, multidistrict litigation DALLAS – When asked by D Magazine researchers to identify the very best lawyers in North Texas for mass torts and multidistrict litigation – the one they’d turn to for their most important lawsuits – peer lawyers in Texas recommended courtroom veteran…

Ben Martin Law Group Lawyer Bailey Grey Named Among Texas Rising Stars for 2022
Ben Martin Law Group lawyer honored by peers for civil litigation DALLAS – For the third year in a row, peer attorneys in Texas have selected Dallas-based trial lawyer Bailey Grey for recognition on the Texas Super Lawyers Rising Stars list of the state’s top young lawyers. Ms. Grey, honored for civil litigation, is a key member of the team at Dallas-based Ben Martin Law…
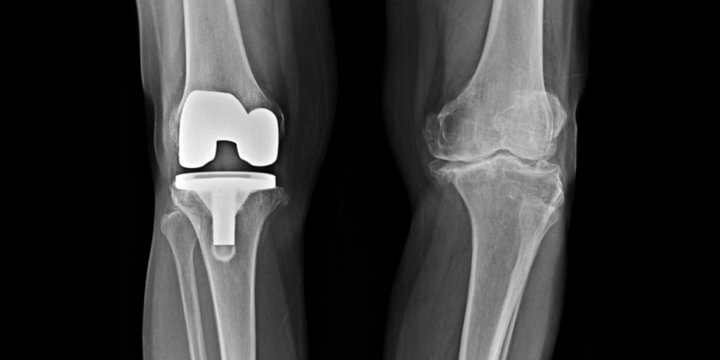
Recall of Over 100,000 Exactech Knee and Ankle Replacements
Many Americans suffer from joint pain in their ankles and knees. Often, the solution is replacement of the joint. But, for those with an Exactech replacement, the device may fail more quickly due to faulty packaging of the component parts. On February 7, 2022, Exactech expanded its recall of all 147,732 arthroplasty polyethylene inserts implanted…

Popular Baby Formulas Linked to Deadly Intestinal Disease
Premature and low birth weight newborns often have trouble nursing or need extra nutrition. Baby foods like Similac and Enfamil are high-calorie, cow’s milk-based products that supply this supplemental nutrition, but do not warn parents of the increased risk that their infant may develop necrotizing enterocolitis (NEC), a serious gastrointestinal disorder. If not caught and…

Contaminated Baby Formula Recalled
Certain powdered infant formula products have been linked to dangerous bacterias, including Cronobacter sakazakii and salmonella. Similac, Alimentum, or EleCare powdered infant formulas manufactured by Abbott Nutrition’s Facility in Sturgis, Michigan have been recalled by the manufacturer, Abbott, due to ongoing investigations by the FDA. Dangerous Bacteria Found in Abbott’s Facility The FDA reported on…
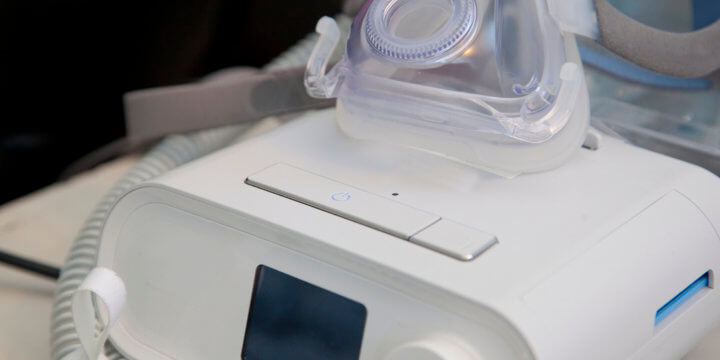
Philips CPAP Recall Update | Expanding Recall of Philips Machines
Since the Philips recall was first announced, it has been expanded several times to reflect a growing number of Philips Respironics ventilators, BiPAP, and CPAP machines, which present serious health and safety risks to users. The following devices have been recalled due to potential health risks: A-Series BiPAP A30 A-Series BiPAP A40 (ventilator) A-Series BiPAP…

Aromatherapy Room Spray Recall
On November 2, 2021, Walmart recalled 3,900 bottles of Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones, which comes in six different scents. The bottles may contain a rare and dangerous bacteria that is capable of causing melioidosis, also known as Whitmore’s disease, which is an infectious disease that can cause…