News Articles
Recall of Over 100,000 Exactech Knee and Ankle Replacements
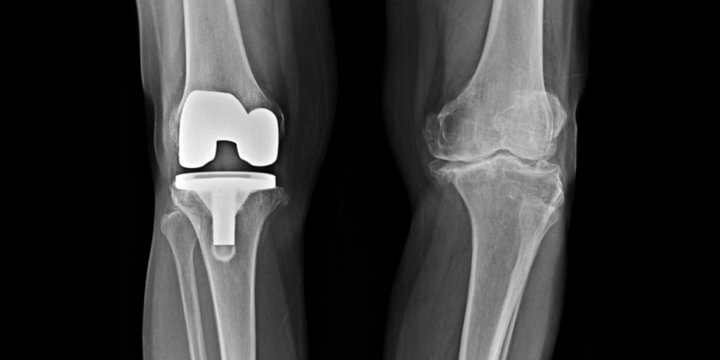
Many Americans suffer from joint pain in their ankles and knees. Often, the solution is replacement of the joint. But, for those with an Exactech replacement, the device may fail more quickly due to faulty packaging of the component parts.
On February 7, 2022, Exactech expanded its recall of all 147,732 arthroplasty polyethylene inserts implanted in the United States. The recall impacts the OPTETRAK®, OPTETRAK Logic (“Logic”)®, TRULIANT®, and VANTAGE® systems manufactured since 2004.
What are Arthroplasty Polyethylene Inserts?
Arthroplasty polyethylene inserts are the plastic liners between the two metallic surfaces in a fixed-bearing knee or ankle replacement. Exactech packaged these inserts in vacuum bags, but later discovered the bags were faulty; the vacuum bags did not fully protect the liners from oxygen and, as a result, the liners were compromised and prone to fail at higher rates. The degradation of the liners reduces the strength and integrity of the liner, accelerates wear, and can cause bone loss, cracks, and fractures. All of these complications often require corrective revision surgery.
Symptoms
Initial signs and symptoms of joint replacement include:
- New or worsening pain;
- Inability to bear weight;
- Grinding, clicking, or other noises;
- New swelling (after the initial surgical incisions have healed); and
- Joint instability.
If you are experiencing these or similar symptoms, you may need to seek medical attention to evaluate whether your knee or ankle replacement is failing.
Our Lawyers Can Help
If you are experiencing problems with an Exactech knee or ankle replacement joint, the experienced attorneys at Ben Martin Law Group may be able to help. Call our firm at (214) 761-6641 for a free, confidential consultation or contact us via email at MBInfo@martinbaughman.com. There is no obligation when you contact us.